Technical Data Sheet, Sulphuric acid
Please request at info@puritysolvents.com
Description
| Catalogue Number | |
| Synonyms | Hydrogen sulfate, 231-639-5, cas 7664-93-9 |
| Overview | Sulfuric acid (95–97%) is a dense, oily, colorless to slightly yellow liquid with no significant odor. It is a strong mineral acid, highly corrosive, and extremely hygroscopic (absorbs water readily). This concentrated form is used in a vast range of industrial processes, from chemical manufacturing to petroleum refining. It reacts violently with water and organic materials, releasing heat. |
Product Information
Sulfuric acid (95–97%) is a highly corrosive, dense mineral acid used extensively in industry, laboratories, and manufacturing. It is strongly exothermic when mixed with water and causes severe chemical burns. Proper handling requires full PPE, ventilation, and caution.
| Formula | H₂SO₄ |
| Molar mass (M) | 98,08 g/mol |
| Density | ~1,84 g/cm³ |
| Boiling point (bp) | ~337 °C |
| Melting point | ~10 °C |
Application(s)
| Chemical Manufacturing |
Produces phosphoric acid, fertilizers, sulfates, hydrochloric acid, and dyes Used in petrochemical processes (alkylation, cracking) |
| Battery Electrolyte | Essential component in lead-acid car batteries |
| Industrial Processing |
pH adjustment and neutralization in wastewater treatment Catalyst in nitration, esterification, and dehydration reactions Used in detergent and explosive production |
| Cleaning and Metal Treatment |
Employed in pickling steel and removing rust or scale Surface prep for electroplating |
| Laboratory Use |
Acid digestion for elemental analysis Dehydration agent and drying substance |
Become a Partner of Purity Solvents
 Partner with us to access the highest quality, REACH-compliant industrial-grade solvents.
Partner with us to access the highest quality, REACH-compliant industrial-grade solvents.Ensure purity, reliability, and compliance for your business. Sign up today and streamline your sourcing with confidence!
 Partner with us to access the highest quality, REACH-compliant industrial-grade solvents.
Partner with us to access the highest quality, REACH-compliant industrial-grade solvents.Ensure purity, reliability, and compliance for your business. Sign up today and streamline your sourcing with confidence!
Safety Information according to GHS
| Hazard Pictogram(s) |  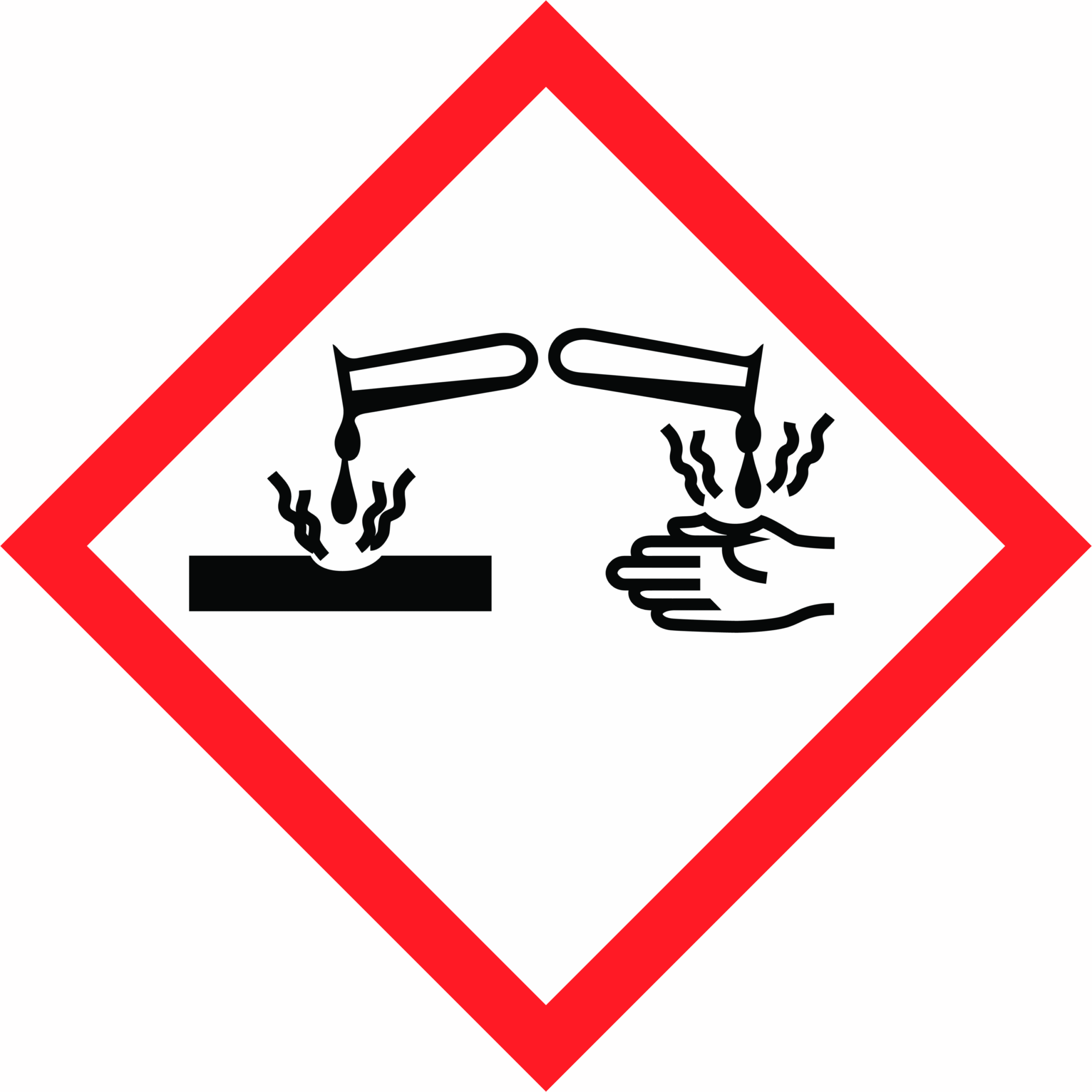 |
| Hazard Statement(s) | H290 May be corrosive to metals H314 Causes severe skin burns and eye damage H335 May cause respiratory irritation |
| Precautionary Statement(s) | Avoid contact with skin, eyes, and clothing Always add acid to water, never the reverse Use chemical-resistant gloves, face shield, and lab coat Ensure adequate ventilation or use fume hood Skin/Eye Contact: Rinse with water for 15+ minutes; seek immediate medical help Inhalation: Move to fresh air; seek medical advice if irritation occurs Spills: Contain and neutralize with sodium bicarbonate or lime |
| Signal Word | Danger |